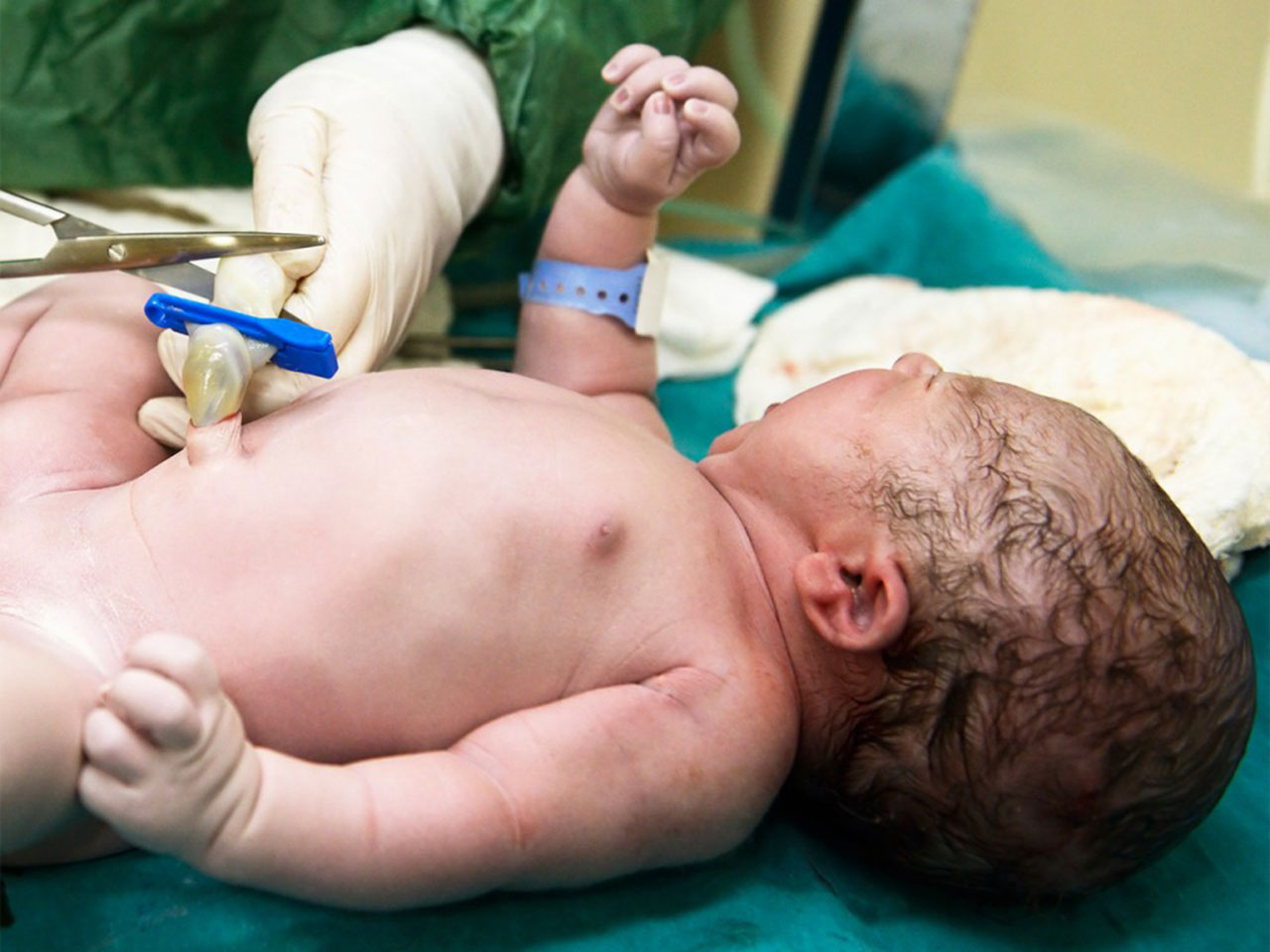
Cord blood is an FDA-approved treatment for nearly 90 diseases including numerous types of deficiencies of the immune system, inherited metabolic disorders, anemias, and malignancies.It’s saved thousands of lives all over the world through over 40,000 transplantations. Nearly all the cord blood transplantations are conducted in patients younger than 18 decades old nevertheless, with progress in regenerative medicine, it’s foreseeable that people of all ages may benefit from this treatment in the not too distant future.
Transplantations are distinguished as either independently (utilized by the infant) or allogeneic (utilized by a fitting sibling or other relative).
Along with the FDA-approved remedies, scientists are looking to ailments cord blood may cure. Promising study that may possibly affect a countless numbers of lives has been ran at treating atherosclerosis, cerebral palsy, grownup stroke and much more. Below is a partial list of disorders now being investigated or treated with stem cells such as those found in cord blood and cord tissue.
About cord banking at Americord

After cord blood has been collected, it’s suspended and could be safely stored for several decades. “The procedure of freezing, known as ‘cryopreservation,’ is also extremely important to keep up the integrity of these tissues,” Wonnacott states.
“Cord blood has to be stored cautiously” You might opt to store your child’s cord blood at a private bank so that it can be accessible if needed later on by your kid or – or even second-degree family members.
Or perhaps you donate the cord blood to a public bank to ensure physicians can use to get a patient that wants a hematopoietic stem cell transplant. FDA regulates cord blood in various ways, based upon the origin, amount of processing and intended usage. Cord blood kept for individual usage, for use at the first – or relatives, which also fulfills other standards in FDA’s regulations, but does not demand the bureau’s consent before use.

Personal cord banks should nevertheless comply with other FDA requirements, including establishment registration and record, current good tissue practice regulations, along with donor screening and screening for infectious diseases (except if cord blood is employed for its first donor).